|
An Acidifying Estuary? The "Other CO2 Problem"
Stephen P. Nash
As CO2 levels are rising in the atmosphere, acidity levels are rising in the ocean, slowing growth rates for coral reefs, oysters, and other shell-building species. What’s happening in the Chesapeake Bay?
Whitman Miller found that oysters did not grow well near Kirkpatrick Marsh on the Rhode River, a study site used by scientists from the Smithsonian Environmental Research Center. Photograph by Michael W. Fincham.
THE SCENE IS MORE THAN A LITTLE ASKEW here in a picnic park along the suburban shore of Maryland's Severn River not far from the Chesapeake Bay. On a drowsy, late-June morning, a high-school kid mows grass in the park, zodiacs and kayaks line the parking lot, and some pleasure craft are bobbing at anchor.
But the picnic tables are laden with an assortment of notebooks and odd tools. And some purposeful folks have unloaded tall green pressure tanks topped with gauges that could be mistaken for a welding kit, now installed in a big plastic chest under the trees. Tubes from their tanks run down into the river and out to a ten-meter square of water. It's marked off by colorful floating foam noodles and attended by two students in snorkel gear.
These are researchers, not welders, and the low-tech look of their apparatus belies the high-stakes questions behind their work. Designed as a kind of time machine, their river setup is meant to simulate Severn River waters as they will be in the future, circa 2050 and 2100. The green tanks are filled with carbon dioxide (CO2), the same gas that now concentrates in the atmosphere as we burn coal, oil, and forests across the planet.
The Severn River project is part of a suite of new and recent research on the effects of rising CO2 levels on the Bay. It is a collaboration between Whitman Miller, a marine ecologist with the Smithsonian Environmental Research Center (SERC), and Tom Arnold, a chemical ecologist with Dickinson College.
The two researchers plan to pump CO2 out of their green tanks and down into that marked-off square of river water. They are doing on a small scale what industrial nations are doing on a global scale by pumping CO2 into the atmosphere in ever-higher amounts. Nearly a third of that airborne CO2 ends up absorbed in the world's oceans, unleashing chemical reactions that raise acidity levels and alter life for many marine species, especially shellfish.
What would happen to oysters in an acidifying Chesapeake? Miller plans to answer that question by placing baskets of young oysters out in that marked-off square of river and then raising CO2 levels. He already knows that oysters don't grow well — at least in a lab — under higher CO2 levels, but lab studies have their limits. "A lab study by its nature is a controlled environment," Miller notes. "The more controlled it becomes, the less realistic."

Coral reefs are falling victim to disease, warming waters, and acidifying oceans. The growth of coral reefs, especially in colder waters, is slowing in the open ocean as levels of CO2 are rising in the global atmosphere. The oceans absorb nearly a third of the airborne CO2, creating chemical changes that raise acidity and even threaten warm-water coral reefs, like this one (top) in St. Croix, U.S. Virgin Islands. Oyster reefs in the Chesapeake Bay (bottom) and other coastal waters could also suffer from growing acidification. Laboratory research indicates that higher levels of CO2 would slow shell growth of young oysters. Photographs: NOAA (top) and Michael Eversmier (bottom).
It turns out real-world experiments also have their limits, as Miller is discovering. Heavy, early summer rains have filled the Severn River with fresh, low-salinity water. Since oysters need moderate salinities to survive, he may have to delay deploying his samples.
The fate of oysters is a high-stakes question because oyster farming is on the rise in Maryland and Virginia, and both states have ambitious plans to rebuild some of the Bay's historic oyster reefs. Those ancient reefs played a key role in the ecology of an ecosystem once known as the "great shellfish bay."
A second question to be answered: what would happen to seagrasses in an estuary with higher CO2 levels? Tom Arnold is planning on taking samples from the meadow of widgeon grass that seems to be flourishing in this part of the river. Heavy rains haven't hurt the widgeon grass, so Arnold can simply walk over and turn the handle on the tank regulator to start pumping CO2 into the Severn River.
Miller and Arnold set up these experiments in the Bay because higher levels of CO2 in the atmosphere are already changing both the climate of the Earth and the chemistry of the open oceans. We think of sky and water as distinctly separate in their vast reaches, but in fact where they meet, they mingle. As CO2 gas increases in the atmosphere, it seeks equilibrium, pressing on the surface of open water and steadily diffusing into it, mixed in by wind and waves. As additional CO2 dissolves into the ocean, it lowers pH and raises acidity. Scientists call this process ocean acidification.
The oceans, as a result, are changing at a faster rate than at any time in the last 300 million years, according to a report published this March in the journal Science. And that's worrisome. An earlier episode of rapid ocean acidification not only brought extinction to many one-celled organisms along the ocean bottom, it also caused the collapse of coral reefs and dissolved all the carbonate plankton shells that once littered ancient seafloors.
If the ocean continues to acidify, seawater could once again become corrosive to calcium carbonate structures, dissolving coral reefs and the shells of many marine organisms. The oceans are already 30 percent more acidic than they were 250 years ago, in the pre-industrial era. According to the Science paper, the current rate of acidification raises the possibility "that we are entering an unknown territory of marine ecosystem change."
What's happening in the ocean could affect the Chesapeake, according to scientists who launched several of the early studies on acidity in the estuary. It's difficult to draw sweeping conclusions, however, since only a handful of studies have been completed so far. If ocean acidification research is in its infancy, as the National Research Council suggests, then the research on estuaries like the Chesapeake is embryonic.
Chemistry 101
How Rising Carbon Dioxide Threatens Shell-Builders
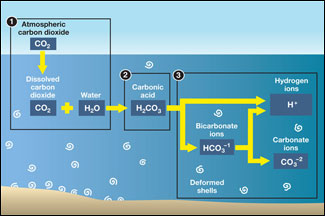
To see an explanation of how excess CO 2 turns ocean waters acidic and how that acidity affects the shells of marine animals in the ocean and in the Chesapeake Bay, click here.
Basic questions, such as what are acidification levels and trends in the Chesapeake, are difficult to answer. To determine acidity, scientists test for pH, the classic scale used to measure the balance between acidity and alkalinity. In an estuary with changing salinities, most pH readings come with an inevitable — and large — margin of error. Checking on acidification, it turns out, is not as easy as dunking some litmus paper in the water.
As a result, "we don't really know how the Bay is going to respond," says ecologist George Waldbusser, whose expertise is in bottom-dwelling organisms. While at the University of Maryland Center for Environmental Science, he was lead author of a recent study that reviewed historical water quality data and tried to reconstruct how acidity has changed in the Bay over the past two decades. The records, he found, were imprecise and tricky to evaluate.
One trend, nevertheless, seems clear: acidity has increased sharply in the Bay's saltier waters — more than can be explained by CO2 in the atmosphere. In the Bay, acidity levels are also driven by other factors, especially by the runoff of nitrogen and phosphorus pollution from farms and sewage. These nutrients generated by human activity lead to explosive growth of phytoplankton in the Bay's waters as photosynthesis converts nutrients and sunlight and CO2 into plant material and oxygen. When all those excess plankton die, however, the decay process sucks oxygen out of the water, creating the Bay's famous dead zones every summer. It also releases a lot of little-noticed CO2. The net effect can be a rise in the Bay's acidity.
"If you say there is no CO2 problem in the Bay, then you have to say there is no oxygen problem in the Bay," says Waldbusser, now a researcher at Oregon State University. "Hypoxia is a byproduct of that oxygen uptake and CO2 release. Those things are linked through biology." For now, that biology and those landborne nutrients create more acidity in the water than airborne CO2 does.
The pteropod, or "sea butterfly," is a tiny sea snail about the size of a small pea. The photos above show what happens to a pteropod's shell when placed in seawater with pH and carbonate levels projected for the year 2100. The shell slowly dissolved over 45 days. Photograph by David Liitschwager, National Geographic Images.
Other trends he found: some historical records show decreases in acidity in the less salty mid-Chesapeake, but surprisingly they also show sharp increases in acidity in the southern Bay where saltier waters have greater buffering power. Waldbusser's explanation: while more plankton dieoffs occur in the mid-Bay, much of the resulting CO2 is transported downstream to the southern Bay.
When Waldbusser applied his findings to laboratory research, he was able to measure some of the impacts of acidification on young oysters and on shell reefs that are critical for the establishment and survival of oyster colonies. His three conclusions: At current average pH levels in some parts of the Bay, the rates of shell growth in young oysters are slowed, creating shells that are likely to be abnormally thin and more vulnerable to predators. In addition, the saltier waters in the southern Bay are likely to become even more acidic and increasingly corrosive to oyster shell. And, finally, some Chesapeake waters, he claims, may already be unsuitable for shell preservation in areas that once supported oyster populations. His analysis, says Waldbusser in his recent paper, should be viewed "cautiously" and followed up with additional research.
If acidification is affecting oysters in the laboratory, could it also be stressing animals and plants out in the waters of the Chesapeake Bay? That's the question that brought scientists Whitman Miller and Tom Arnold to the Severn River last summer. According to Miller, "Nobody's really been looking."
That may be changing. Arnold designed a project to examine the impact of rising CO2 levels on the Bay's underwater grasses by pumping CO2 gas into an underwater research plot that held widgeon grass. His focus was photosynthesis and the protective compounds that grasses use to ward off predators and disease.
Perhaps more CO2 in the water could improve prospects for the Bay's underwater seagrasses. Under ideal conditions, CO2, sunlight, and water all combine to drive photosynthesis for these submerged grasses, a key support system for the health of the Bay. Since the 1970s, unfortunately, conditions have been less than ideal, and nearly all seagrass species have declined dramatically. The Bay's waters remain so clouded with silt pollution that the grasses no longer get much light.
Arnold's findings from last summer's fieldwork were less than hopeful. Starved for light, seagrasses, he found, are unlikely to take advantage of added CO2. There may not be enough light to help CO2 stimulate photosynthesis, but there is enough acidification, unfortunately, to erode the grasses' protective compounds. By the end of the season, his research established that a rise in CO2 levels was followed by a sharp reduction of those protective compounds.
"I've been doing this for about 20 years now, and it's the largest change I've ever seen," Arnold said recently, summing up last year's experiment. "Everything we see so far tells us that it's not going to be so great for the seagrasses after all."
There were no findings at all, however, about oysters in the Severn River. Miller had planned to chart the physical development of juvenile native oysters as they matured over the summer, sitting in a basket amid plumes of elevated CO2 and rising acidification. When heavy rains kept salinities too low for oyster survival, Miller decided he would have to postpone his river experiment.
Chemistry 102
The pH Scale: Yardstick for Acidity
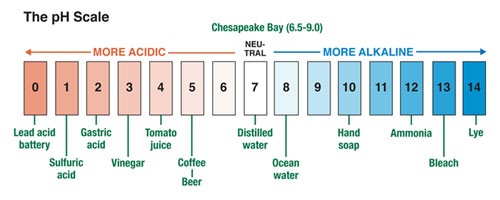
The pH scale — that staple of introductory chemistry courses — is the measuring stick that scientists use to gauge acidity in the Chesapeake Bay. The scalae normally runs from 0 to 14; the lower the number, the greater the acidity. A pH of 7, the middle point, is regarded as neutral pH. That is the reading for distilled water, or pure H2O with nothing dissolved in it. more . . .
Based on his earlier lab findings, Miller suspects that rising acidification will make it more difficult for the Bay's oysters to form calcium carbonate structures — shells and skeletons. When he charted shell growth under a variety of CO2 regimes, he found that higher CO2 brought slower shell growth. At levels predicted for the year 2100, he found that the shell area of maturing native oysters decreased by 16 percent, and their calcium content by 42 percent.
Similar failures have already shown up among ocean calcifiers such as sea butterflies, some planktons, and corals. In oyster hatcheries along the northwest Pacific Coast, large dieoffs of oyster larvae have been linked to upwellings of acidic ocean water (see separate article, Shell Game). In the Chesapeake, acidification could also affect plankton as well as some or all of the many mollusk species that grow shells (see separate article, Crab vs. Oyster).
Not all scientists agree, however, that rising acidity levels will be a threat to the Bay's ecology. This brand of optimism, for example, is sometimes heard: because plants and animals in the Chesapeake ecosystem are adapted to variability, they will not be sensitive to added increments of acidification. Native species may in a sense be pre-adapted, from an evolutionary standpoint, to at least some degree of future change in acidity.
But shellfish calcification in particular is a long-term process of integration, says Richard Feely, a senior scientist who studies ocean acidification with the National Oceanic and Atmospheric Administration. "When they calcify, they calcify all day long, so what matters is that average value, over the course of time," he says. "That's what affects their rates. So yeah, you see a lot of variability, but you want to know what the overall trend is."
The overall trend is clear, at least according to scientists like Waldbusser, Miller, and Feely. The change in atmospheric CO2 is permanent — it will be with us for hundreds or thousands of years — and it is all in one direction.
How would small oysters like these (above) grow in more acidic waters? Researchers Whitman Miller (right) and Tom Arnold (left) wanted to pump CO2 from tanks into a patch of the Severn River to see whether small oysters and widgeon grass would grow well in an acidifying river. Photographs by Stephen P. Nash.
And it's that long-term, one-way direction that may pose a future threat to the Chesapeake Bay. "What we're most concerned about is a baseline shift of the whole system," Miller says. "You can imagine if we increase the CO2 just a bit more, what happens is you will still have variability, but you'll shift that variability to a different place on the pH scale. And it may not take a tremendous amount before many parts of the cycle of variation are outside the tolerance level of the organisms. If we have a shift in the baseline, that could mean bad news for lots and lots of organisms, no matter how adaptable they are."
The idea is worth dwelling on. You can think of the Chesapeake's normal cycles as rising and falling within certain limits, as if they were inside a picture frame. Aquatic life has adapted to live within those boundaries, but survival rates fall off near the edges. If you move the whole picture frame incrementally — as atmospheric CO2 rises, for example — survival chances diminish. The Miller-Arnold experiments are moving that frame, simulating acidification that is an evolving threat to the already sorely challenged, unstable health of the Bay.
Because their projects simulated CO2 in the years 2050 and 2100, they may give the illusion that we have plenty of time to figure things out and ease the threat. The changes, though, are incremental — they won't arrive all at once, a few decades out. "Fifty to 100 years is not a very relaxed schedule, actually, given the magnitude of the problem," Miller says. "We're fighting against a time clock here."
The fight, however, has to focus first on the immediate, and crucial, job of throttling back on land-based pollution threats, especially farm runoff and sewage flows (see separate article, Should We Regulate Acidity in the Bay?). For now, these nutrients generate more CO2 in estuaries like the Chesapeake than atmospheric CO2 does. According to Waldbusser and Miller and other scientists who study acidification in the Chesapeake, these nutrients are the strongest factors that may move the baseline, tipping acidification levels beyond the tolerances of life in the Bay.
"We've altered coastal ecosystems in ways that have affected that carbonate chemistry and already pushed the system to levels that are predicted for [the open ocean] a hundred years from now, or even further into the future," adds Waldbusser. "I think we're worse off than the open ocean."
Stephen P. Nash teaches journalism at the University of Richmond and has written for Bioscience, The New York Times, The New Republic, The Scientist and The Washington Post. His most recent book is Millipedes and Moon Tigers: Science and Policy in an Age of Extinction.
|