
Tracking sample after sample, Allen Place turned up a toxic dinoflagellate in his laboratory that now shows up at fish kills all along the Mid-Atlantic and beyond. Photograph by Michael W. Fincham.
|
By Jack Greer
There is another scenario for the fish kills blamed on Pfiesteria in the Pocomoke River and other parts of the Chesapeake in the late 1990s — and for the fish kills that have happened since.
That alternate narrative emerges from the work of professor Allen Place and others at the Center of Marine Biotechnology (COMB), part of the University of Maryland Biotechnology Institute. Here is what Place thinks happened. In August 1997 an algal bloom formed at the mouth of the Pocomoke River, common in the nutrient-rich waters of the Chesapeake Bay. Most likely this crowd of algae contained what scientists call cryptophytes. Cryptophytes are not toxic. They are, in fact, a major food source for algae eaters, like menhaden.
When menhaden gathered to feed on the bloom, dinoflagellates also showed up. Not unusual. Dinoflagellates are whip-tailed single-celled organisms that may use photosynthesis to process energy from the sun, like plants, or they may feed on other organisms — or they may do both. For many feeding dinoflagellates, cryptophytes are a favorite prey.
As the menhaden fed on algae, these dinoflagellates passed through their gills. A toxin — likely evolved by dinoflagellates to help them catch their food — began to attack the gill tissue of the fish. With oxygen levels in the shallow river mouth already low, the menhaden struggled to breathe.
Then the algal bloom crashed and dissolved oxygen plummeted, consumed in the breakdown of dead algal cells. With their gills compromised and oxygen now scarce, the fish began to die in droves.
Dead and dying fish drew dormant Pfiesteria out of their cysts in the sediment and they began to feed — a process well described by scientists like JoAnn Burkholder at North Carolina State University. Living up to their reputation as the "cell from hell," Pfiesteria gorged on this banquet of fish tissue.
While this scenario does not differ widely from what many surmised before, in Place's version Pfiesteria did not kill the fish. He believes the toxin that damaged the gills of menhaden came from another dinoflagellate, one that shares Pfiesteria's diminutive 10-micron size, one that now goes by the name of Karlodinium veneficum.
Veneficum is Latin for poisonous, or in our vernacular, toxic.
Flash back to 1996, when Maryland scientists first picked up the trail of this Chesapeake Bay fish killer. The earliest clue came with a phone call to Dan Terlizzi at his home near Westminster, Maryland. Terlizzi is a Sea Grant Extension specialist and an expert on algae, water quality, and aquaculture. The phone call came from Tony Mazzaccaro, the owner of HyRock fish farm in Somerset County.
Mazzaccaro had invested heavily in a system of ponds and in a large stock of hybrid striped bass. He had had good luck in finding a market for his fish, but he was having problems with water he drew from the Manokin, a river located just north of the Pocomoke on Maryland's Eastern Shore. His stripers had gone off their feed and were swimming at the surface. It didn't sound good.
Terlizzi made the 170-mile drive down to Somerset County where he found a fish pond gone reddish brown, the color of what many call a mahogany tide. Under the simple microscope they had at HyRock he could see a dense bloom, with several species of dinoflagellates swimming around in their characteristic swirl. And the fish were suffering.
Terlizzi advised treating the pond with a pesticide, and Mazzaccaro decided to use copper sulfate, a compound he'd used successfully before. Terlizzi nodded and drove home.
By the next morning the dinoflagellates were dead. So were the fish — some 15,000 almost-market-sized hybrid striped bass. Terlizzi and Mazzaccaro needed to know what had killed them (see The Copper Connection).
Many of the dinoflagellates swarming under HyRock's microscope were about 10 microns or so, the size of a droplet of mist and tiny even for microscopic phytoplankton. Dinos this small look pretty much alike under a conventional microscope, so they sent samples to Wayne Coats at the Smithsonian Environmental Research Center (SERC) for further analysis. Coats identified several small dinoflagellates, including one that looked like Gyrodinium estuariale, originally discovered in Woods Hole, Massachusetts in the 1950s and widely assumed to be nontoxic.
Coats then forwarded the samples to a leading expert on identifying and categorizing marine dinoflagellates, Karen Steidinger at the Florida Fish and Wildlife Conservation Commission. Using an electron microscope, Steidinger detailed the physical structure of these tiny dinos, and reported that she found several species. One of them was "armored" with cellulose plates and positively identified as Pfiesteria, a species she had helped to describe with JoAnn Burkholder and her colleagues in North Carolina. Her micrographs also revealed a "naked" species without plates, Gyrodinium galatheanum, the species we now call Karlodinium.
While the spotlight at that time fixed on Pfiesteria, Terlizzi and others noted that in the HyRock fish kill cells of the species now known as Karlodinium outnumbered those of Pfiesteria a hundred to one. In a paper delivered at a conference in Vigo, Spain in June 1997 Terlizzi detailed the toxic algal bloom at HyRock. He described the presence of Pfiesteria, but questioned whether it had caused the fish kill. After all, the bloom had been dominated by this other species. While others remained focused on Pfiesteria, Terlizzi began to suspect that the other species — Karlodinium — might have been to blame.
Public concern about sick fish and sick people (see Medical Mystery Trip) soon brought more focus and more funds to the world of dinoflagellate research. By 1998 Place and others had won a five-year grant to study toxic algae from the National Ocean and Atmospheric Administration (NOAA) and the National Institute of Environmental Health Sciences (NIEHS).
When Place put out a call for graduate students to help with the research, Jonathan Deeds saw the ad. Deeds worked at the Aberdeen Proving Grounds on microbes and water quality issues and had been contemplating a return to graduate school. He had followed the news about Pfiesteria and saw a chance to serve on the front lines of scientific research. He took it. Place interviewed him and a week later hired him.
Deeds hit the ground running, but he and Place faced a serious roadblock. Though they had cultures of Pfiesteria, they could not readily get hold of Pfiesteria cultures that had been certified as toxic. What they did get were cultures of a co-occurring dinoflagellate, one quite similar in size to Pfiesteria that would make a good control. For them, as it turns out, this proved a remarkable piece of scientific luck and an important turning point in their story.
The dinoflagellate in question was the same one found at HyRock — Karlodinium. It came from Aishao Li, a graduate student at the University of Maryland Center of Environmental Science (UMCES) who worked with Diane Stoecker, a leading dinoflagellate expert. Li sent them two samples of Karlodinium: One came from the Chesapeake Bay and one from the deadly HyRock bloom.
When Place and Deeds set about growing and testing cultures of Pfiesteria and Karlodinium, they soon found that the apparently nontoxic cultures of Pfiesteria did not kill fish at normal environmental densities. But Karlodinium, the co-occurring dinoflagellate, did — again and again. When Deeds ran one Karlodinium culture lightly through a centrifuge to get a better concentrate, the resulting fluid not only killed fish larvae, it virtually "dissolved" them.
Both Karlodinium cultures — the one gathered from the Bay and the one taken from HyRock — proved toxic. Getting the toxin from this single-celled dino was relatively easy, since Karlodinium is a "naked" dinoflagellate, without cellulose plates like those of Pfiesteria and other "armored" species. The toxin oozes out when the cell passes through a filter or through the gills of a fish.
Place and his team now turned their attention away from Pfiesteria and focused on Karlodinium. Could this be the fish killer? They ran more experiments, using fish blood (hemolytic) assays, which proved to be very sensitive to the toxin. To dissect the purified compound, they used precise instruments that analyze the mass and color spectrum of molecules.
Place and Deeds established a dose-response curve for karlotoxin, an essential step in documenting the toxicity of any compound. They know how much toxin it takes to kill a fish, what tissues are targeted (such as gill tissue) and they can demonstrate this lethality in a matter of minutes, showing characteristic tissue damage. Their results match well the early descriptions of the toxin and its lethal effects first provided in the 1950s by British researchers Mary Parke, Dorothy Ballantine, and B.C. Abbott. These early researchers found that the toxin would kill mice in two to four minutes and a two-foot dogfish in three hours. It also caused respiratory problems for one asthmatic worker.
Fifty years ago, researchers did not have the tools now available to characterize the toxin's molecular structure. That classification depends on the sophisticated techniques of modern analytical chemistry and it requires a lot of purified toxin to work with. Place and his team would spend a year producing two milligrams, holding nearly four billion cells of Karlodinium veneficum. And then they would spend a second year purifying the sample.
Place eventually sent samples to two different chemists to determine the structure of karlotoxin. One sample, sent to Jeffrey Wright at the University of North Carolina at Wilmington, came from the Chesapeake Bay. The second sample, sent to Mark Hamann at the University of Mississippi, came from waters south of the Bay. The toxin in both samples had a structure resembling another class of marine toxins called Amphidinols, metabolites produced by a marine dinoflagellate of the genus Amphidinium — a group previously associated with fish kills.
Place believes he can now describe how the toxin works at the cellular level. Think of a cell as a tiny battery, he says, with its electrical charge isolated from its surroundings by a membrane (the cell wall). Karlotoxin destabilizes the cell's ionic balance by puncturing the membrane, a strategy similar to what white blood cells in humans do when they attack a virus or other invader.
|

|
|
|
|
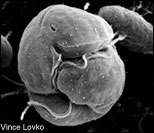
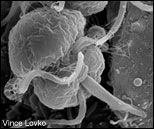
Focused first on Pfiesteria, Allen Place soon identified Karlodinium as the producer of a potent toxin. Called a "naked" dinoflagellate, Karlodinium (top left) lacks the armored cellulose shell and feeding tube (peduncle) of Pfiesteria (lower left). Both single-celled organisms are about the size of a droplet of mist (10 microns). Micrographs of Karlodinium and Pfiesteria by Vince Lovko, Virginia Institute of Marine Science. Photograph of Alan Place by Michael W. Fincham.
|
|
Place theorizes that karlotoxin lands on a cell, chemically penetrates the membrane, and then draws sterols (like cholesterol) to itself to form a molecular barrel-like pore through the cell wall. Once the pore penetrates the cell wall, ions and water flow in and out of the cell, and the battery is discharged. The cells literally explode.
Can karlotoxin be found in nature — specifically at the site of fish kills? For Place, answering that question was as important as identifying the structure and behavior of the toxin. After monitoring karlotoxin levels in the Chesapeake Bay for the last three years, he thinks the answer is yes. Every year since 1998 there have been fish kills attributed to Karlodinium along the Atlantic coast, with karlotoxins found in samples taken from Delaware, Maryland, North Carolina, South Carolina, Georgia, and Florida. Deeds and Place note that today's more accurate methodologies have turned up both Karlodinium and karlotoxins at virtually every dinoflagellate-related fish kill in mid-Atlantic estuaries over the past seven or eight years — including the June 2007 fish kill in Weems Creek, Annapolis. Pfiesteria, on the other hand, has appeared less often and in far fewer numbers.
Asks Deeds, "Is this a coincidence?"
But what about the fish kills in the Pocomoke in 1997? Does Deeds believe that Karlodinium killed those fish? There's no way to know for sure, he says. We just didn't collect enough information at the time. Molecular probes that provide more definitive identification did not come until later — in 1998 for Pfiesteria and in 2000 for Karlodinium. He notes that no toxins had even been identified in what was called "Gyrodinium galatheanum" back then.
Wayne Coats at the Smithsonian agrees that reconstructing what happened in 1997 may prove impossible. Coats feels that research has pointed to at least two toxic dinoflagellates at the site of Pocomoke fish kills, Pfiesteria and Karlodinium, and maybe that's all we need to know.
Place has heard this argument but remains convinced that Karlodinium is the real culprit responsible for fish kills. He notes that researcher Holly Bowers, now also at COMB, has run molecular probes on archived DNA samples taken near fish kills back in 1998 and she's found evidence of Karlodinium there. Questions remain about the roles of Karlodinium, Pfiesteria, and other co-occurring microbes, including bacteria. But there is one organism, Place argues, that repeatedly turns up at estuarine fish kills worldwide, with a toxin that we can reliably measure, identify, and verify in the laboratory. That organism is the toxic marine dinoflagellate first found in the 1950s. The one we now call Karlodinium veneficum.
![[Maryland Sea Grant]](/GIFs/h_footer_mdsg.gif)