Microbes Can Teach
Contents
Up Polluted Groundwater
By Erica Goldman

Its red letters are now faded and rusty, but the old "BOWL" sign still towers above an abandoned parking lot in Towson, Maryland, a reminder of a time when teenagers flocked to Fair Lanes and customers brought their rumpled shirts and slacks to Kings Cleaners, a dry cleaning operation that stood next door. Now the bowling alley is a roofless cement hull. Yellow and blue paint peels off the sides, rows of steel girders rust beneath the sky. In the old bowling alley's vast interior, gleaming new construction equipment rests idle — the only sign that the site will soon be redeveloped.
Deep below the paved lot surrounding the abandoned bowling alley, and well out of sight, a contaminated groundwater plume slowly creeps toward Mine Bank Run, a tributary of Gunpowder Falls. Kings Cleaners has vanished entirely — except for a square gravel stain in the center of the parking lot. But its chemical signature lingers strong and clear. Twenty feet beneath the former dry cleaning operation, tetrachloroethene (PCE), a widely used cleaning solvent, persists in the groundwater at high concentrations, measured at 16,400 parts per billion (ppb) in 2004, more than 3,000 times the legal limit of 5 ppb set by the U.S. Environmental Protection Agency.
PCE is a suspected human carcinogen and difficult to clean up. It can contaminate groundwater, a grave concern in rural areas that rely on wells for their drinking water supply. When this compound percolates close to the surface along with groundwater, it can intrude into the soil, releasing volatile, noxious gases. Short-term PCE exposure can cause dizziness, headaches, and problems with balance. Over the long term, PCE exposure has been linked to cancers of the esophagus, bladder, and blood.
Kings Cleaners closed in 2000, after continuous operation for 32 years. In 1998, the Maryland Department of the Environment (MDE) began a series of environmental assessments that lasted for years — testing contaminant levels in the groundwater, in soil gases, and in nearby residential wells — to determine whether remediation would be necessary prior to any future development.
In October 2005, MDE decided to allow limited industrial and commercial development at the site, as long as the groundwater remains untouched. Tests had deemed the soil gases free of PCE. So the chemical poses no immediate threat to future occupants of the site. The agency issued what is known as a "No Further Requirements Determination" to the current property owner, meaning that no remediation steps would be mandated. But no residences can ever be constructed on the site. No wells can ever be dug.
Sites like the former Kings Cleaners exist all over the United States. According to a study by the State Coalition for Remediation of Dry Cleaners, 75 percent of all active dry cleaning facilities are contaminated (some 17,000 sites nationwide), and that doesn't include sites that have been abandoned.
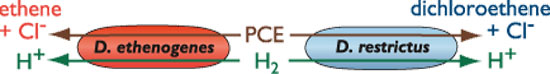
Are there tools to clean up these toxic messes? Scientists know that there is one species of bacteria — only one — that can break down PCE and its chemical cousin TCE (trichloroethylene), a compound with a similar chemical structure used in metal degreasing and paint stripping. To this "bug" (Dehalococcoides ethenogenes), chlorinated solvents such as PCE and TCE are the air that they breathe. So shouldn't cleaning up PCE-contaminated sites be as simple as providing this toxin-breathing bug unfettered access?
It's not that easy. In nature, a single species of bacteria rarely acts alone. Microbes live in intricate consortia and interact with other organisms — in the marine environment these include algae, corals, and sponges. Different bugs may play complementary roles, or they may compete fiercely. Scientists have long worked to harness the power of microbes to clean up human messes. Now, more and more, they are looking to the ecology of bacteria for clues about how to tackle tough challenges — like erasing the environmental signatures of industrial pollution.
Jennifer Becker leaves her office in the Biological Resources Engineering Department at the University of Maryland in College Park and walks briskly across campus to the Plant Sciences Building to give an invited lunchtime seminar. She sets up her computer and plugs into the room's audio-visual system as the previous class files out.
Her first Power Point slide features two potato-sized shapes, thousands, maybe millions of times the size of the bacteria they represent. Their stick figure arms wear boxing gloves. Facing each other, they square off, ready to duke it out.


![[Maryland Sea Grant]](/GIFs/h_footer_mdsg.gif)